B-ONE INTERACTIVE, INC.
ARTHREX SYNERGY UHD4 SYSTEM
Usability testing of an endoscopic medical device system (hardware & software)
OVERVIEW
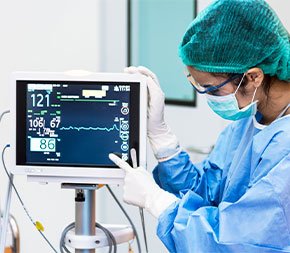
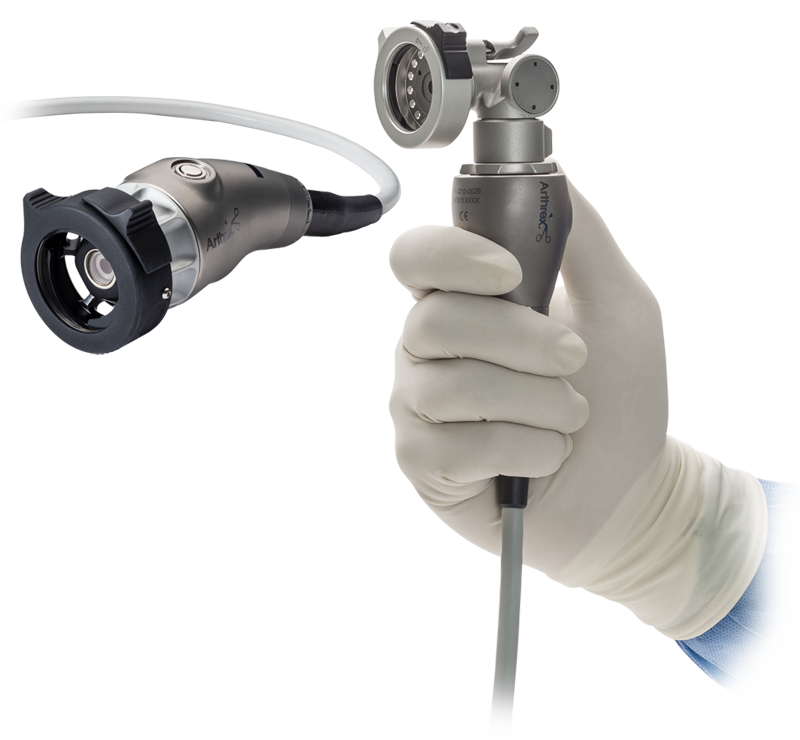
The SynergyUHD4 imaging platform is a commanding video system offered by Arthrex, used for exploratory surgery with endoscope devices.
It features an ultra HD 4K programmable camera head, LED “xenon-bright” light source, an image management system, and fiber optic video over IP integration, all in one tablet-controlled device. The network-based system allows live video streaming to any authorized remote viewer during the use of an endoscope in real time.
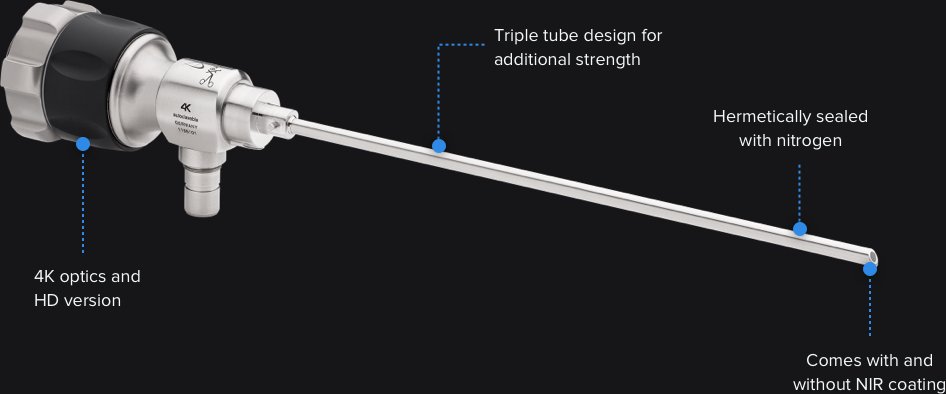
This device includes hardware (endoscope, camera heads, cords, a tablet, a large viewing screen, different attachments, and remote control buttons on the camera head. It also includes software, which is the entire viewing system on the tablet which offers surgeons the ability to record video, take images, save data, view data, and schedule exploratory surgeries.





MY ROLE
Role: UX Design & Human Factors Research Consultant
Team: Arthrex (a medical device company) & B-One Interactive, Inc. (a design agency)
Timeframe: 2020-2021
USERS
Because we were doing usability testing with a medical device, it was crucial that we test it with actual users of the product, for safety purposes. We wanted very accurate results from users to make sure we could prepare a risk analysis of the product.
The users for this project were surgeons, nurses, and lab technicians in the Southern California area.
METHODS
-
Participants were shown in detail how to power on, set up, use, and break down the device. They were able to ask questions. Then they took a 1 hour break and came back to test the device themselves.
-
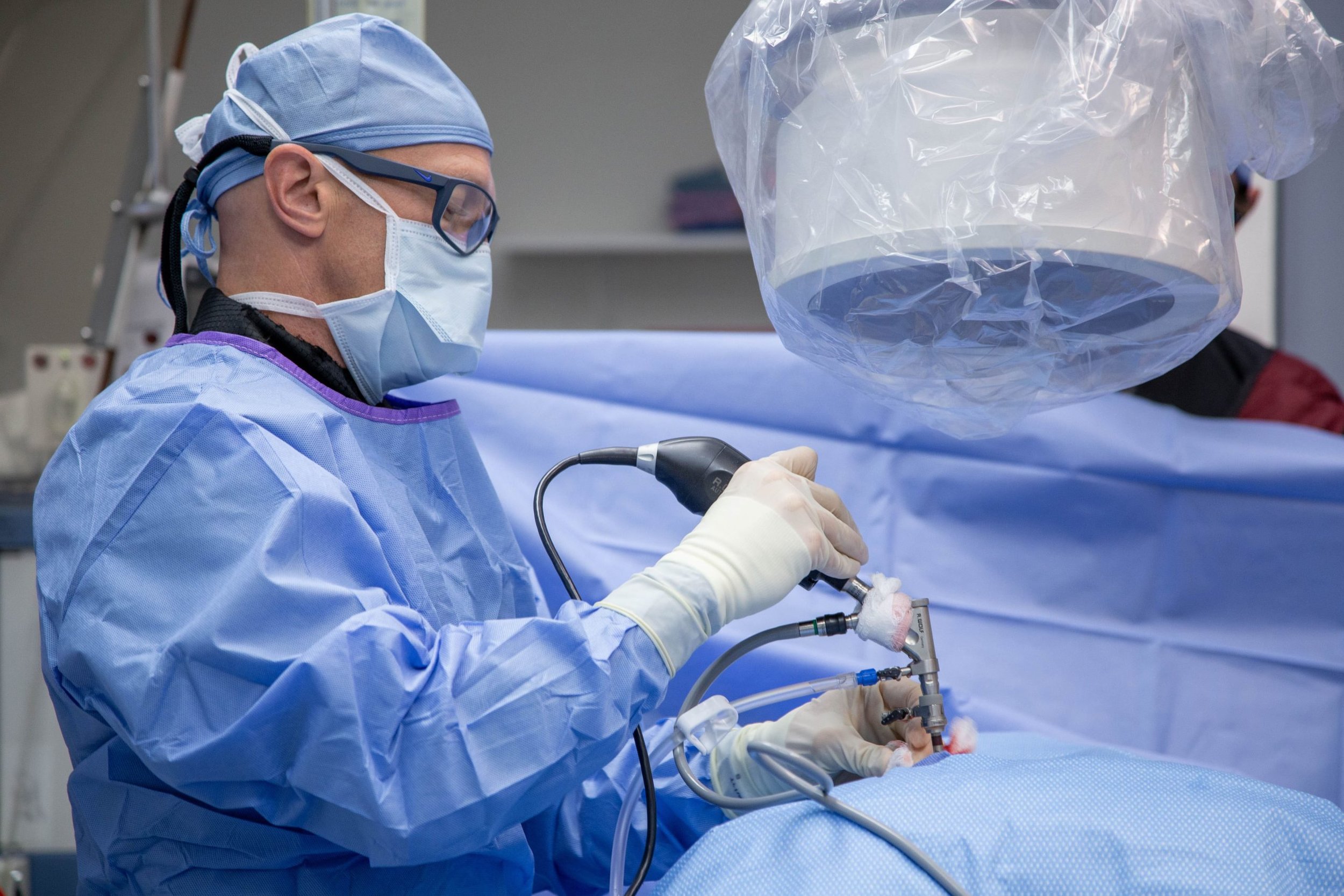
B-One Interactive worked with Arthrex, a medical device manufacturing client, to test the usability of this endoscopic device. 10 surgeon/lab technician/nurse pairs were created at random and used for this study. Each pair had never met or worked together before. Pairs were asked to set up the device for use, follow a series of tasks with the device on the mannequin, and then shut down the device and put it away. The setting was a realistic operating room with all of the necessary appliances and materials.
-
After the completion of the usability test, pairs sat down in a room with a researcher and were asked to answer a series of questions about their experience. Questions targeted performance of the device, ease of use, ability to complete tasks, etc. They were also asked what they thought of the device in terms of aesthetic and what they would change if given the opportunity.
INSIGHTS
-
It was discovered that the device needed to have a very specific weight in order to be functional for its users. Surgeons spend all day in the OR holding devices. Their hands get very fatigued having to stay steady and hover above a patient’s body for multiple hour surgeries, back to back.
-
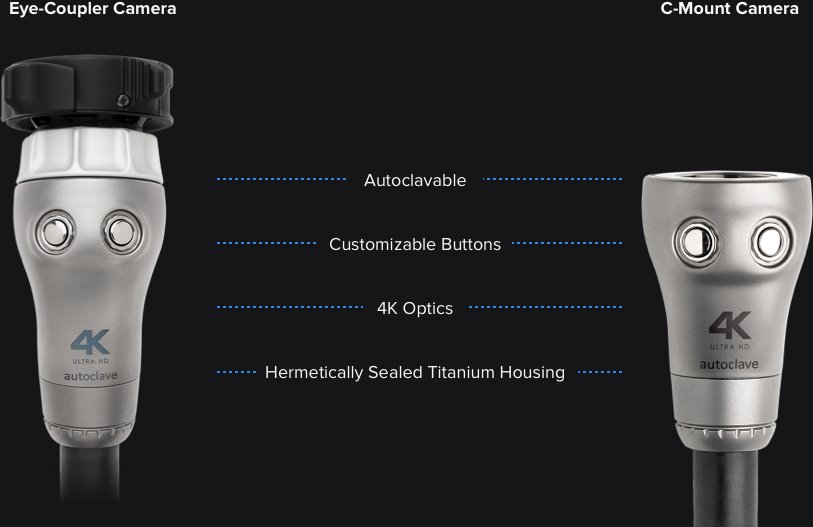
Detailed findings about the device’s remose control buttons were provided to the client. Participants had differing mental models of how to assign button preferences and how to actuall use the buttons.
-
We provided detailed findings about the device’s touch screen component and how it was used and perceived by participants who must maintain sterility.
-
Insights about the laser component of the device were provided including ease of use, perception, connecting and disconnecting, etc.
-
Insights about how users interpreted and used the instructional manual were provided to the client, with recommendations of how to improve it to be more user friendly.
IMPACT
-
Detailed reporting of the device’s usability was given to the client’s cross functional teams
-
Recommendations for design improvements based on participant data was given to design and engineering teams for both the hardware and software components
-
A new and improved instruction manual was given to the client based on findings from this study.
-
New UI designs of the software component were created and tested.
-
Further research was completed as a result.
-
Findings were used to create a version 2 of the device.